Background:
Thrombophilia in children is characterized by hypercoagulability and increased frequency of thrombotic events (Young G, et al. Circulation. 2008;118:1373-1378; Kenet G, et al. Circulation. 2010;121:1838-1847). Recently, phase IIb/III clinical trials in children with venous thromboembolism (VTE) have reported the non-inferiority of dabigatran etexilate (DE) versus standard of care (SOC) for the treatment of acute VTE (Albisetti M, et al. ISTH 2019, Abstract OC 57.3), and a favorable safety profile for DE in secondary VTE prevention in children with persistent VTE risk factor(s) (Brandão LR, et al. Blood. 2020;135:491-504).
Aims:
To perform a subgroup analysis evaluating the efficacy and safety of DE for the treatment and secondary prophylaxis of VTE in children with thrombophilia in the phase IIb/III DE clinical trials.
Methods:
In the open-label, phase IIb/III DIVERSITY trial (NCT01895777), children aged from birth to < 18 years (yrs) with an objectively confirmed VTE diagnosis (by imaging studies) initially treated with unfractionated heparin or low-molecular-weight heparin were randomized (2:1) to receive up to 3 months of DE or SOC. Primary composite efficacy endpoint: complete thrombus resolution and freedom from VTE recurrence, or VTE-related death. Safety endpoints included bleeding events (BEs). The open-label, phase III, secondary VTE prevention trial (NCT02197416) treated children aged from > 3 months to < 18 yrs with DE for up to 12 months or less, if the identified VTE clinical risk factor resolved. Eligible children had an objectively confirmed diagnosis of VTE treated with SOC for ≥ 3 months, or had completed DE or SOC treatment in DIVERSITY and had an unresolved clinical VTE risk factor requiring further anticoagulation. Primary endpoints included VTE recurrence and BEs. Thrombophilia status was confirmed according to the definitions used by the local experts.
Results:
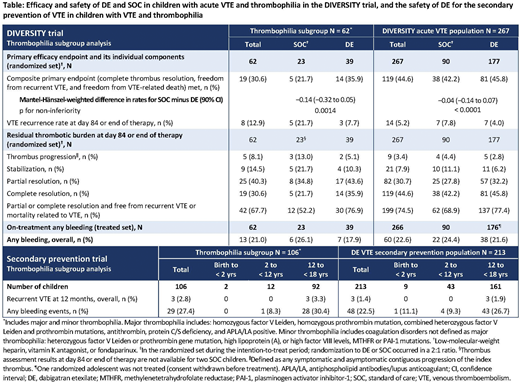
In DIVERSITY, 23.2% of children had thrombophilia; demographics were comparable to the overall population, although they were slightly older (mean [standard deviation] age 13.2 [4.9] vs 11.1 [6.1] years in the overall population; p = 0.005). In children with thrombophilia, DE was found to be non-inferior to SOC for the primary endpoint (similar to the overall population), and more children treated with DE achieved the composite primary endpoint than with SOC (SOC 21.7% vs DE 35.9%; Mantel-Hänszel-weighted difference in rates for SOC minus DE [90% CI] −0.14 [−0.32 to 0.05]; p for non-inferiority = 0.0014), similar to the overall population (Table). Regardless of treatment, VTE recurrence appeared higher in children with thrombophilia than in the overall population, with numerically fewer VTE recurrences reported by children with thrombophilia treated with DE (7.7%) versus SOC (21.7%), although this was not significantly lower (p = 0.13). Numerical differences in residual thrombotic burden between SOC vs DE seen in the overall population appeared to be amplified in children with thrombophilia. Numerically fewer children with thrombophilia treated with DE reported thrombus progression (SOC 13.0% vs DE 5.1%) or stabilization (21.7% vs 10.3%), while more reported partial (34.8% vs 43.6%) or complete thrombus resolution (21.7% vs 35.9%). In the thrombophilia subgroup, BEs appeared to be lower in children treated with DE (SOC 26.1% vs DE 17.9%), while in the overall population BEs with SOC and DE were comparable. In the secondary VTE prevention trial, children with thrombophilia were also slightly older versus the overall population (mean [standard deviation] age 14.1 [3.6] vs 12.8 [4.6] yrs; p = 0.006). In this larger subgroup of children, rates of recurrent VTE at 12 months appeared to be higher in the thrombophilia group (2.8%) compared to the overall population (1.4%) (Table), with BEs largely comparable (27.4% and 22.5%, respectively).
Conclusions:
Unsurprisingly, numerically more children with thrombophilia appeared to report VTE recurrence, and in DIVERSITY thrombus progression/stabilization also seemed higher compared with the overall population. Compared with the overall populations, these subgroup analyses showed consistent results for DE in children with acute VTE and thrombophilia in the DIVERSITY trial, along with a favorable safety profile of DE for secondary VTE prevention.
Brandao:Boehringer Ingelheim: Other: Member of a paediatric expert working group. Tartakovsky:Boehringer Ingelheim: Current Employment. Albisetti:Boehringer Ingelheim: Other: Member of a paediatric expert working group; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees. Bomgaars:Boehringer Ingelheim: Other: Member of a paediatric expert working group. Chalmers:CSL Behring: Honoraria; Shire/Takeda: Honoraria; Boehringer Ingelheim: Other: Member of a paediatric expert working group; Grifols: Honoraria; Roche: Honoraria; Sobi: Honoraria; Bristol-Myers Squibb: Honoraria. Mitchell:Boehringer Ingelheim: Other: Member of a paediatric expert working group. Luciani:Boehringer Ingelheim: Other: Member of a paediatric expert working group. Lvova:Boehringer Ingelheim: Honoraria. Simetzberger:Boehringer Ingelheim: Current Employment. Sun:Boehringer Ingelheim: Current Employment. Gergei:Boehringer Ingelheim: Current Employment. Brueckmann:Boehringer Ingelheim: Current Employment. Halton:Boehringer Ingelheim: Other: Member of a paediatric expert working group.
dabigatran etexilate in paediatric VTE
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal